MetalLigand and MetalMetal Bonding Lecture Notes
Awesome Prices & High Quality Here On Temu. New Users Enjoy Free Shipping & Free Return. Come and check All Categories at a surprisingly low price, you'd never want to miss it.

The 10 Best 3M Metal Bonding Adhesive Life Maker
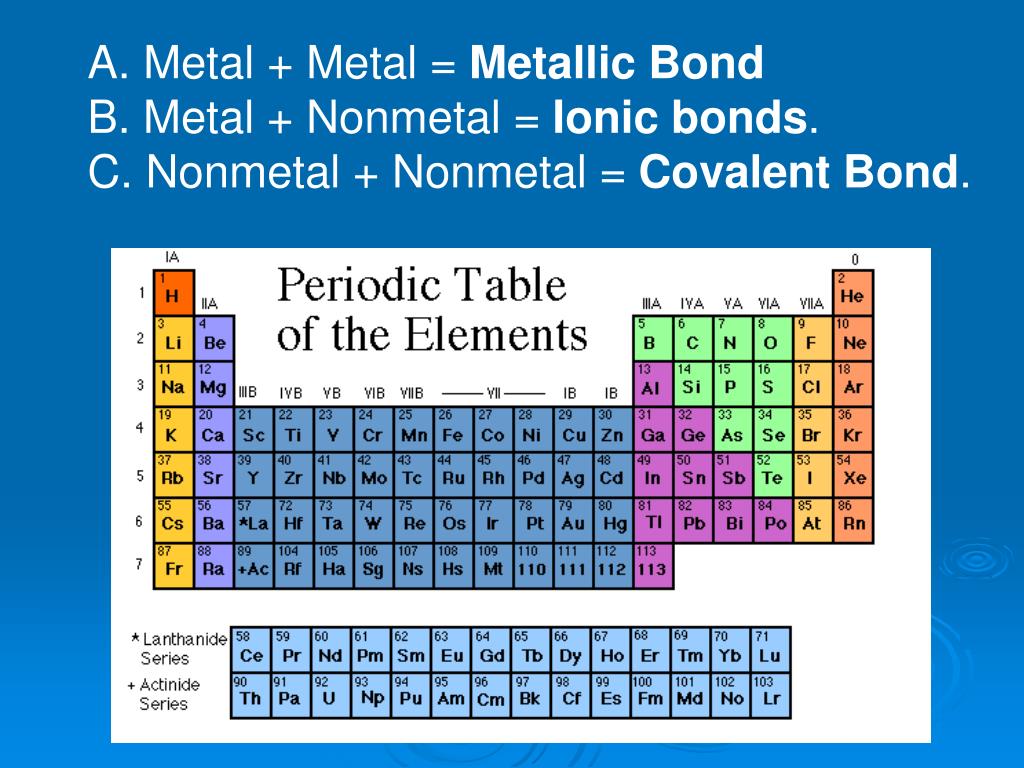
About Transcript One way to predict the type of bond that forms between two elements is to consider whether each element is a metal or nonmetal. In general, covalent bonds form between nonmetals, ionic bonds form between metals and nonmetals, and metallic bonds form between metals. Created by Sal Khan. Questions Tips & Thanks

Metallic Bond — Formation & Compounds Expii
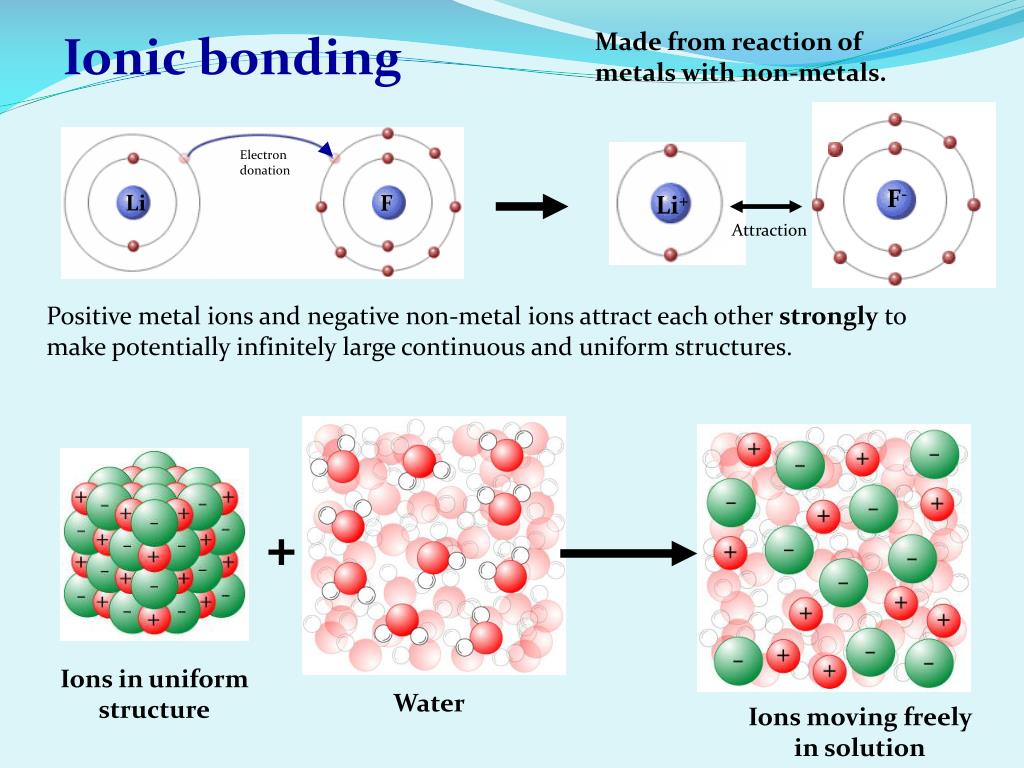
When metals react with non-metals, electrons are transferred from the metal atoms to the non-metal atoms, forming ions. The resulting compound is called an ionic compound. Reactions.

(PDF) MetalMetal Bonding
When a metal and a non-metal react with each other, the metal atom donates its outer electrons, and the non-metal atom gains these electrons to form an ionic bond between them. Why does a metal atom donate its outer electron and a non-metal atom gains electrons? How is an ionic bond formed? Let us answer these interesting questions in this article.

Metallic Bonding Definition, Properties, Examples, Diagram
While there are only seventeen non-metals on the periodic table a few common examples include oxygen and nitrogen which account for most of the air that we breathe, along with a few other gases like neon or the chemical compound carbon dioxide. The non-bonding helium, neon, radon, argon, xenon, krypton, and oganesson, also known as the noble.

The 10 Best 3M Metal Bonding Adhesive Life Maker
Chem1 (Lower) 9: Chemical Bonding and Molecular Structure
PPT Chapter 1 Chemical Bonding PowerPoint Presentation, free download ID1482753
High. Formation: A covalent bond is formed between two non-metals that have similar electronegativities. Neither atom is "strong" enough to attract electrons from the other. For stabilization, they share their electrons from outer molecular orbit with others. An ionic bond is formed between a metal and a non-metal.
PPT Structure and bonding in metals PowerPoint Presentation, free download ID5242010
In a metallic bond, each metal atom is surrounded by lots of other metal atoms, and they all share their valence electrons. When two oxygen atoms bond, they become a molecule and don't interact much with other molecules.

Metal to metal bonding methods
Covalent bonding occurs in most non-metallic elements and in compounds of non-metals. Metallic bonding occurs in metallic elements and alloys. Forming Ions. An ion is an atom (or group of atoms) with a positive or negative charge, formed by either losing or gaining electrons.

Metal Bonding Capabilities The Atlas Group
https://engineers.academy/A tutorial to explain how ionic bonds form between metals and non-metals.In order to become stable, metal atoms lose electrons. Non.

The 10 Best 3M Metal Bonding Adhesive Life Maker
Abstract. Metal-linker bonds serve as the "glue" that binds metal ions to multitopic organic ligands in the porous materials known as metal-organic frameworks (MOFs). Despite ample evidence of bond lability in molecular and polymeric coordination compounds, the metal-linker bonds of MOFs were long assumed to be rigid and static.

metallic bonding occurs between atoms of best tricktaking card games
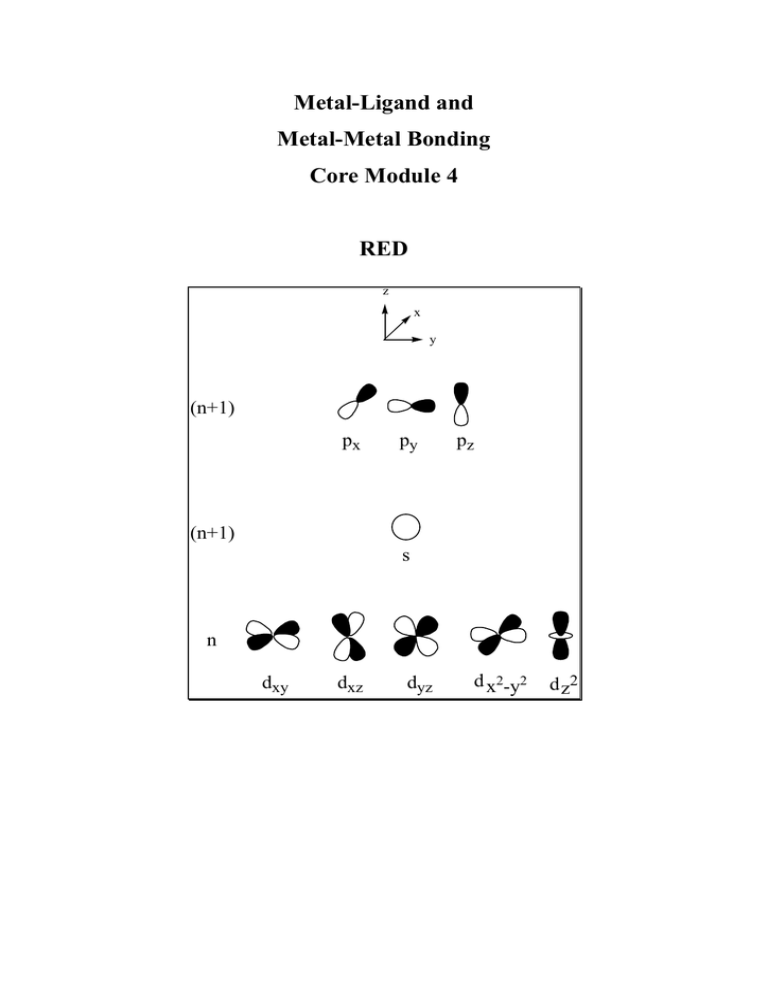
Figure 11.1.1 Molecular orbital overlap of metal d-orbitals forming M-M bonds. If we define the M-M bond axis as the z-axis, then two d z2 -orbitals can overlap in σ-fashion to form a bonding and an anti-bonding σ-molecular orbital. A d xz, and a d yz can overlap with another d xz and another d yz in π-fashion to form two degenerated bonding.
Chem Metals and NonMetals Scientific Tutor
2.4: Metallic Bonds. In the early 1900's, Paul Drüde came up with the "sea of electrons" metallic bonding theory by modeling metals as a mixture of atomic cores (atomic cores = positive nuclei + inner shell of electrons) and valence electrons. Metallic bonds occur among metal atoms. Whereas ionic bonds join metals to non-metals, metallic.
0.9 Bonding in metals and metalnonmetal salts By OpenStax Jobilize
There are 3 types of bonding: Ionic bonding - when metals and nonmetals give/take electrons from each other. Covalent bonding - when nonmetals share electrons with other non-metals. Metallic bonding - when metals form a lattice of positive ions surrounded by a sea of electrons. The more electrons that are shared/taken/lost, the stronger the bond.

Covalent Bonding Between Two Non Metals (Atomic Bonding Mechanisms) YouTube
Bonds between metals and non-metals Ask Question Asked 10 years, 2 months ago Modified 6 years, 1 month ago Viewed 27k times 3 My teacher in college says that bonds between metals and nonmetals are ionic. $ \ce {Metal - Metal} $ $\Rightarrow$ Metalic bond $\ce {Non metal - Non metal}$ $\Rightarrow$ Covalent bond

savvychemist Periodicity (2) Melting and boiling points of the elements of Period 3
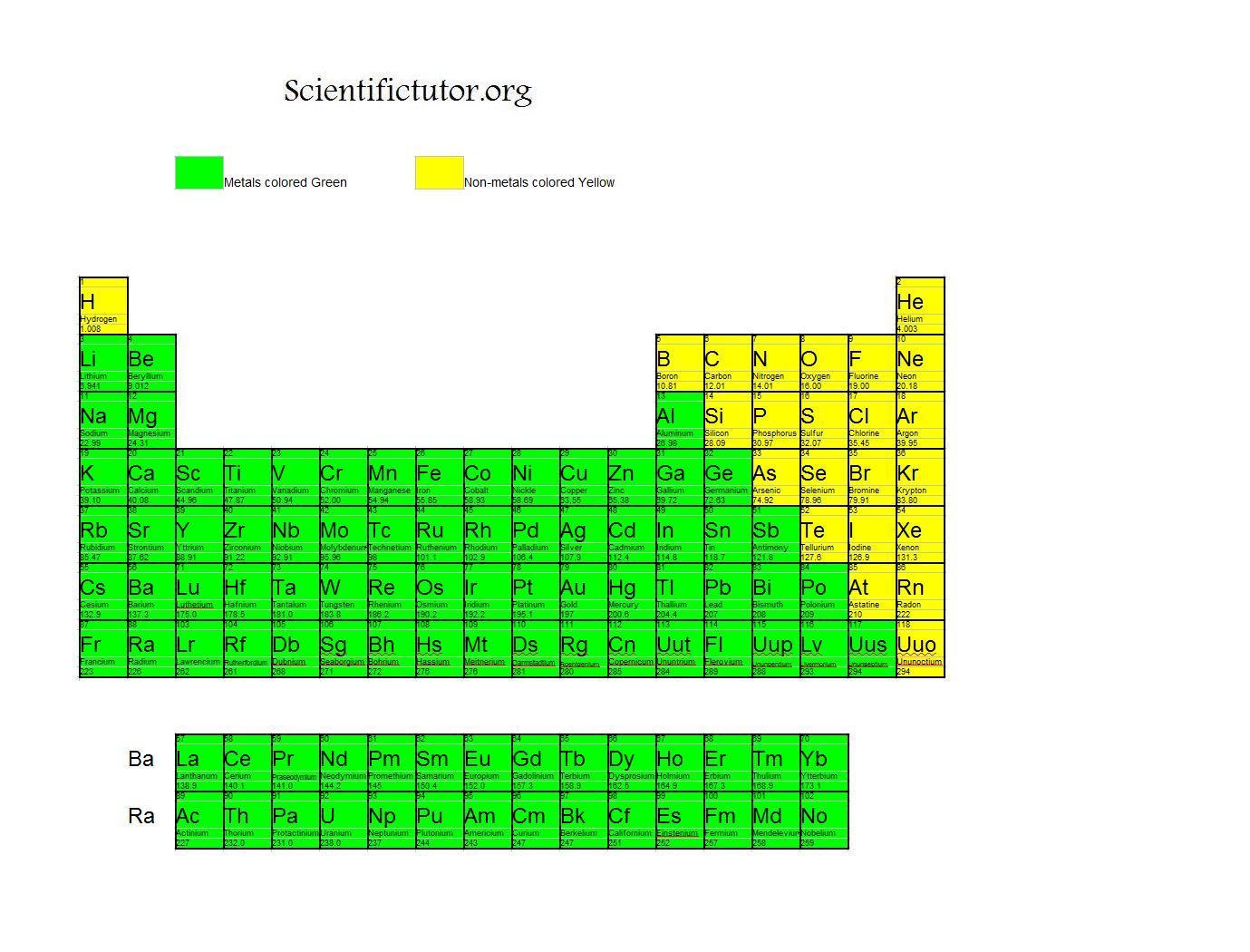
In general, you find metals on the left-hand side of the periodic table and non-metals on the right-hand side. Notice that: there are 118 known elements. there are many more metals than non-metals. The red zig-zag line approximately separates metals from non-metals. Metalloids have some properties of metals and non-metals.